加载中...
共找到 15,256 条相关资讯

Tom Sosnoff, Founder and CEO of LossDog and former CEO of Tastytrade, says retail traders are driving market moves, rotating away from parts of the Mag 7, with near-term volatility and profit-taking likely after triple witching.
After the Fed acted to cut the Federal Funds Rate by a quarter point on Wednesday, 10 December 2025, the S&P 500 went on to strike a new record high of 6,901.00 on Thursday, 11 December 2025. But the index retreated the next day as new fears arose about the outlook for earnings of firms making big AI-technology investments.

Appetite for Israeli technology innovation has remained undiminished this year, with a surge in acquisitions and IPOs led by Alphabet's $32 billion purchase of Israeli cybersecurity company Wiz, PwC Israel said on Monday.

Frank Luntz, pollster and political strategist, joins 'Squawk Box' to discuss the debate around affordability, bringing cost down vs. bringing wages up, impact on next year's midterm elections, and more.
Hedge funds sold Hong Kong and Japanese stocks last week, Goldman Sachs said in a note, just before the tech-heavy Hang Seng and Nikkei indices fell in the last two trading sessions on worries over over-inflated tech values.
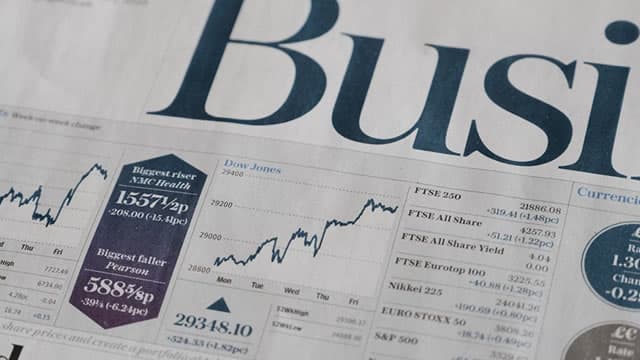
U.S. stocks settled lower on Friday, with the S&P 500 falling over 1% during the session following a renewed tech-led sell-off. The S&P 500 fell 0.6% last week, while the 30-stock Dow gained 1.1% on the week.

CNBC's Melissa Lee reports on the 5 things to know on December 15, 2025.

During times of turbulence and uncertainty in the markets, many investors turn to dividend-yielding stocks. These are often companies that have high free cash flows and reward shareholders with a high dividend payout.
Delayed jobs and inflation data come out this week, Supreme Court tariffs decision looms, SpaceX could serve up a feast in 2026, and more news to start your day.
What matters in U.S. and global markets today
As investors stare down the start of 2026, one thing is clear: the playbook that worked over the last decade won't cut it in the next. Between stubborn inflation fears, a ballooning US national debt and relentless innovation in digital finance, the lines between Wall Street, Washington and Web3 are blurring fast.

The MoneyShow Chart of the Day shows the S&P 500 Equal Weighted Index. It hit a fresh high last week, too - indicating we have broader participation in this latest leg up.
The most oversold stocks in the consumer staples sector presents an opportunity to buy into undervalued companies.

“They are tuned in to friends and colleagues, who are losing jobs, cognizant of shifts in company agendas and financial worries.”

The year started with a bang, with President Donald Trump taking office and launching tariffs that caused shockwaves in the stock market, leading investors to sell, sell, sell, then buy, buy, buy. Supply-chain issues have pumped up the cost of everything from groceries to clothing to cars.

Markets delivered strong returns in 2025 for diversified investors sticking to core assets, with Tech and non-US equities outperforming. I maintain exposure to US large-cap Tech (VOO, QQQM) and European equities (FEZ, FLGB, SCHF), expecting continued momentum and value diversification.
Global stock markets were mixed to start the week, with falls across Asia even as European bourses opened up and U.S. stock futures pointed to a modestly higher open.

U.S. markets head into a data-packed week with CPI, Non-Farm Payrolls, and Fed commentary in focus, alongside key earnings from Micron, FedEx, and Nike.
European markets are set to open in the green following Friday's A.I.-led stock sell-off ahead of a variety of data prints and rate decisions later in the week.
Watch more: Wage to Wallet: WorkWhile's Alan Armen The consumer still drives the U.S. economy, but a new Wage to Wallet Index™ report suggests that a growing share of that spending depends on platforms workers turn to when wages wobble.